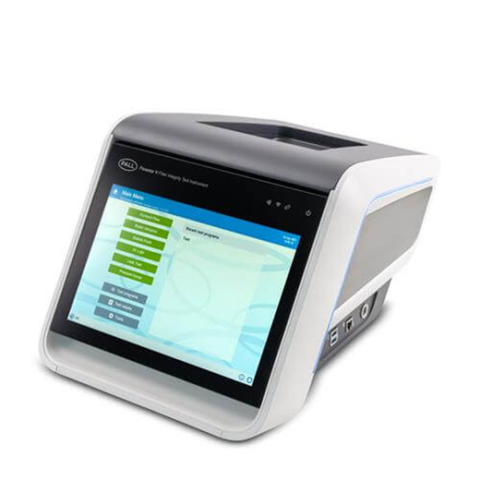
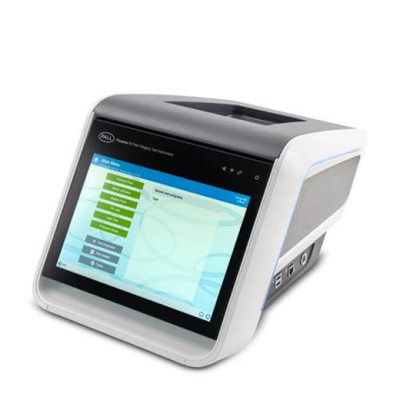
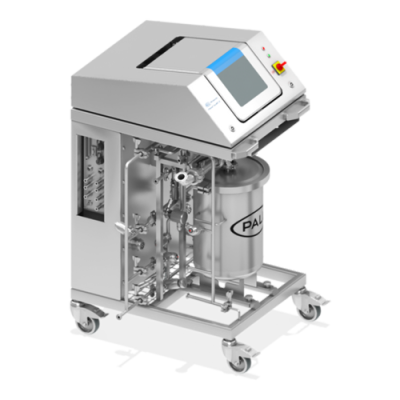
Palltronic Flowstar V 过滤器完整性测试仪
高精度、快速完成过滤器完整性测试,同时满足最严苛的法规要求。
产品详情
Palltronic Flowstar V 仪器提供精确的过滤器完整性测试解决方案,具备先进的自动化能力及简化的网络集成,为用户节省时间并提升工艺效率。主要特性包括:
- 直接流量测量技术,测试更快速、结果更精准,并可基于可溯源的流量基准进行校准
- 可存储测试参数与配方,减少设置时间和输入错误
- 直观的大尺寸触摸屏界面,提升可读性和数据录入效率
- 符合 GAMP5 质量标准与 21 CFR Part 11 法规要求,防护等级 IP54
- 便于随时审查,审计追踪功能完整记录所有审计所需数据
- 高级特性电子记录与电子签名、条码扫描器以及测试重复功能等
- 可选网络集成与自动化接口:OPC-UA、PROFIBUS、DeviceNet™、Profinet、串口、PAS-X MES、WiFi 2.4 GHz
补充软件、硬件及附件
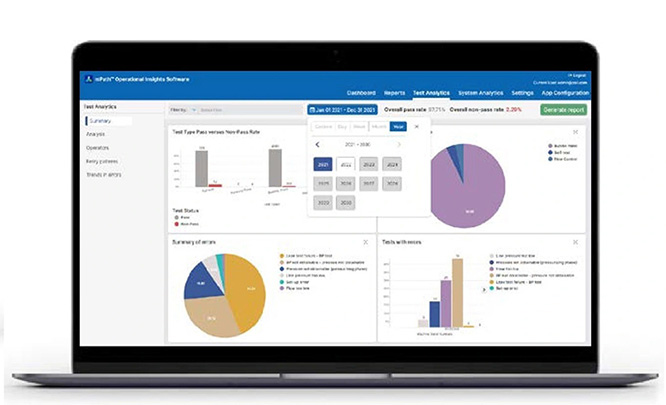
mPath™ 运营洞察软件 (OIS)
相关产品
培训课程
本讲座 – 颇尔MVP系统应用操作示例的所有视频列表
相关文档下载
相关产品推荐
资料下载
搜索未找到相关记录,请换个查询条件尝试。

 收藏
收藏 询价
询价