A unique three-layer construction contributes to outstanding throughput, high flow rates, and the superior retention of bacteria and mycoplasma during the filtration of process fluids.
The Ultipleat modular cartridge pleating design in combination with a narrow core ensures high effective filtration areas, small foot-print filter systems, and improved process efficiencies in these0.1 micron capsule filters. A complete validation package supports the assurance of safety within your process.
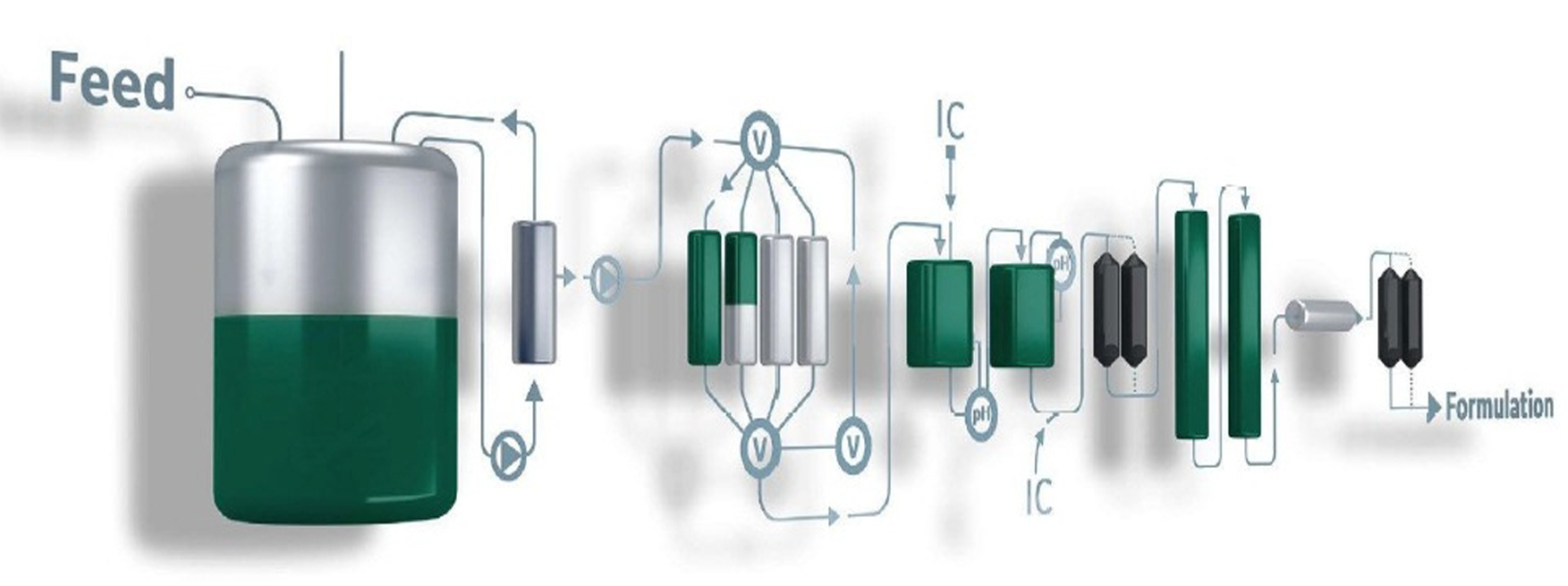
Kleenpak Nova capsules are designed for use in medium to large scale production environments (100 L > 1000 L), often selected by the end-user following scaling studies using smaller Kleenpak capsule formats. With the AB style cartridge format at its core, this capsule filter style can be supplied with the most comprehensive range of filter media.
Key Features and Benefits
Encapsulated format for higher flexibility, minimized cleaning, and low installation costs
Ultipleat filter construction allows for high flow rates and smaller filtration systems
Small core design for more area
Asymmetric built-in prefilter for highest dirt-holding capacity
> 10 log A. laidlawii and M. orale retention
High flow rate
Low protein and preservative binding
Low extractables
Designed for autoclaving or SIP under wet or dry conditions
Quality Standards
Manufactured for use in conformance with cGMP
100% integrity tested
ISO 9000 Certified Quality System
Meets USP Biological Reactivity Test, in vivo, for Class VI-121°C Plastics
Every filter tested during manufacture. Test correlated to microbial retention
Certificate of Test provided includes:
Fabrication Integrity
Bacterial Retention
Materials of constructions
Effluent quality for cleanliness, TOC and Water Conductivity, pH and Pyrogens

 收藏
收藏 询价
询价