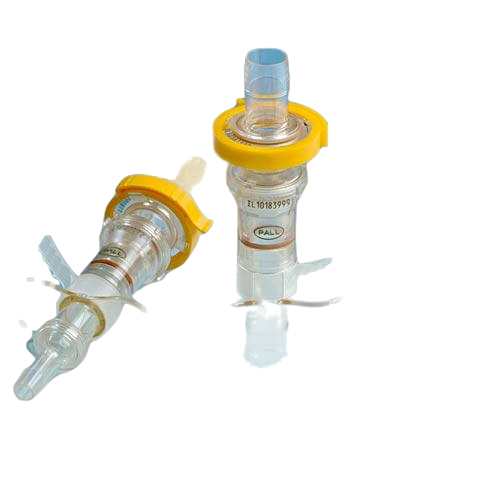
Kleenpak™ sterile disconnectors
Convenient and secure sterile disconnection of flexible tubing
产品详情
Kleenpak™ sterile disconnectors, a Pall Life Sciences product, provides an easy to use, secure method for the sterile disconnection of flexible tubing assemblies even in an uncontrolled environment.
The disconnectors can also be used in processes involving single-use systems as well as hybrid systems. The disconnector maintains sterility of the fluid path before and after disconnection. The disconnector consists of a joined male and female unit, to fit tubing with 6 mm (1⁄4 in.), 9.5 mm (3⁄8 in.) or 13 mm (1⁄2 in.) ID. Kleenpak™ sterile disconnectors can either be autoclaved up to 130 °C or gamma irradiated up to 50 kGy.
相关产品
培训课程



































 收藏
收藏 询价
询价